Medical device certification acc. to Regulation (EU) 2017/745 (MDR)
TABLE OF CONTENT
DESCRIPTION OF SERVICE
Inquiry means any preliminary question related to the conformity assessment process and the activities of the notified body. The inquiry received is reviewed by the responsible employee of the medical device certification department in order to confirm the product qualification proposed by the manufacturer. If the product meets the definition of a medical device according to the requirements of MDR, the client is asked to provide a copy of a completed Preliminary questionnaire .
The questionnaire is the basis for obtaining more detailed information about the applicant’s company and products and is one of the bases for setting the fees for the certification process. If the questionnaire does not contain all required information, the applicant is asked for its completion.
Based on the information provided in the questionnaire, NB 1023 verifies the proposed qualification and classification of the product in question. In case of a different opinion on the qualification or classification of the product, the applicant is referred to contact the relevant competent authority with a request for an official opinion. Subsequently, the opinion shall be submitted to NB 1023 for review.
If NB 1023 confirms the correctness of the qualification and classification of the products and if the products fall within the scope of NB 1023 notification, NB 1023 will prepare a price offer based on the information provided in the preliminary questionnaire, including the project plan. For Class IIa and IIb (non-implantable) medical devices that are described by multiple separate technical files, the project plan may include a sampling plan for the assessment of the technical files. The project plan shall include an assessment of the technical documentation, initial audits and surveillance activities (periodic audit, unannounced audit, extraordinary audit), and shall be set for a maximum period of 5 years. The applicant will receive a price offer which includes the project plan (description of the planned activities) within 10 calendar days of the correct completion of the preliminary questionnaire. If the product does not fall within the notification scope of NB 1023, the inquiry is rejected.
The fees for the services offered by NB 1023 are listed in the document Fees for the conformity assessment. .
If the client rejects the price offer or does not confirm it within 14 days, the inquiry is rejected, otherwise the NB 1023 administrator verifies whether the manufacturer has entered the necessary information (according to Article 31(1) of the MDR) in the Eudamed system and obtained a single registration number (SRN). In the case of Class III devices and Class IIb implantable devices (except sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips and connectors), the NB 1023 administrator shall verify that the manufacturer has provided the Basic UDI-DI to the Eudamed electronic system in accordance with the provisions of Article 29(1) and (3) of the MDR.
If the client has fulfilled the registration obligations, he will receive a form of Application for conformity assessment from the NB 1023 administrator for completion (alternatively, the form can be downloaded from the link below). The duly completed and signed application form must be sent to the ITC either in paper or scanned form within 7 days. At the same time, the manufacturer is asked to sign the General Framework Agreement GFA-MDR (an agreement form is sent to the manufacturer by the NB 1023 administrator or again can be downloaded from the link below). If the client has already concluded a GFA-MDR with NB 1023 (for previous conformity assessment processes), it is not necessary to conclude this agreement again.
The application received by the ITC is reviewed for formalities (completion of all data, dating, signature) and in case of deficiencies the applicant is invited to complete or correct the application.
Once the application has been reviewed, the project is registered and the manufacturer receives an access name and password to the application WIDAR off-line, which serves as an attachment to the application containing data on the device and quality system under assessment and also as a communication tool between the manufacturer and NB 1023 for the purpose of reviewing the documentation. The generation of WIDAR off-line login data can also be done on receipt of a scanned application. The original request must be received by ITC no later than the date of delivery of the technical file.
The manufacturer shall complete all required data in WIDAR off-line and deliver the complete technical file to ITC within 30 days of receipt of the login data. Once the required data has been completed and the cycle is closed, the data entered is locked and the manufacturer is only able to view the WIDAR offline, at the same time the WIDAR is made available to NB 1023 personnel who can add comments on individual items.
If the manufacturer fails to make the WIDAR off-line available within the above timeframe, he is notified to arrange a remedy within an additional 7 days. If even this deadline is not met, the application will be rejected.
The use of WIDAR off-line is described in the Handbook for clients - WIDAR.
The technical documentation to be sent by the manufacturer following the completed data in the WIDAR off-line shall be prepared in accordance with the requirements of Annex II and Annex III of the MDR.
A separate application for conformity assessment including the completed WIDAR application shall be submitted for each class of medical device.
Upon submission of a duly completed and signed conformity assessment application, including the completed WIDAR application, NB 1023 shall check the completeness of the submitted documents and review aspects that could lead to the rejection of the application.
The reasons that could lead to refuse an inquiry:
- product is out of the notification scope of NB 1023,
- the documentation contains facts that could lead to a conflict of interest and compromise impartiality or independence,
- inapplicability of the conformity assessment procedure chosen by the manufacturer to the device concerned,
- the application contains several classes and / or categories of medical devices for which a separate application must be submitted,
- the limited capacity of the NB 1023 staff to perform all the required activities does not ensure the prerequisites for certification within an acceptable time interval,
- the manufacturer has not truthfully informed that he has submitted a previous application to another notified body and the application has been withdrawn or rejected,
- the manufacturer has failed to remedy deficiencies in the application of which he has been informed,
- the manufacturer has not made the annex to the application (WIDAR) available to the ITC within the required time after submission of the application and has not responded to the ITC's urging, so that the application is incomplete,
- the manufacturer has failed and/or refused to sign the current version of the GFA-MDR.
If no obstacles are identified during the assessment of the application that prevent the initiation of the conformity assessment process, the manufacturer will receive a copy of the application signed by NB 1023 and a contractual relationship will be established for the conformity assessment project as per the application. Based on the overall project plan, a long-term contract is issued covering all activities performed as part of the initial certification and for the entire validity of the certificate (maximum 5 years). The contract shall also include a reference to the subcontracts under which NB 1023 will carry out the individual activities. The conclusion of the long term contract and the subcontract formally and technically initiates the conformity assessment process.
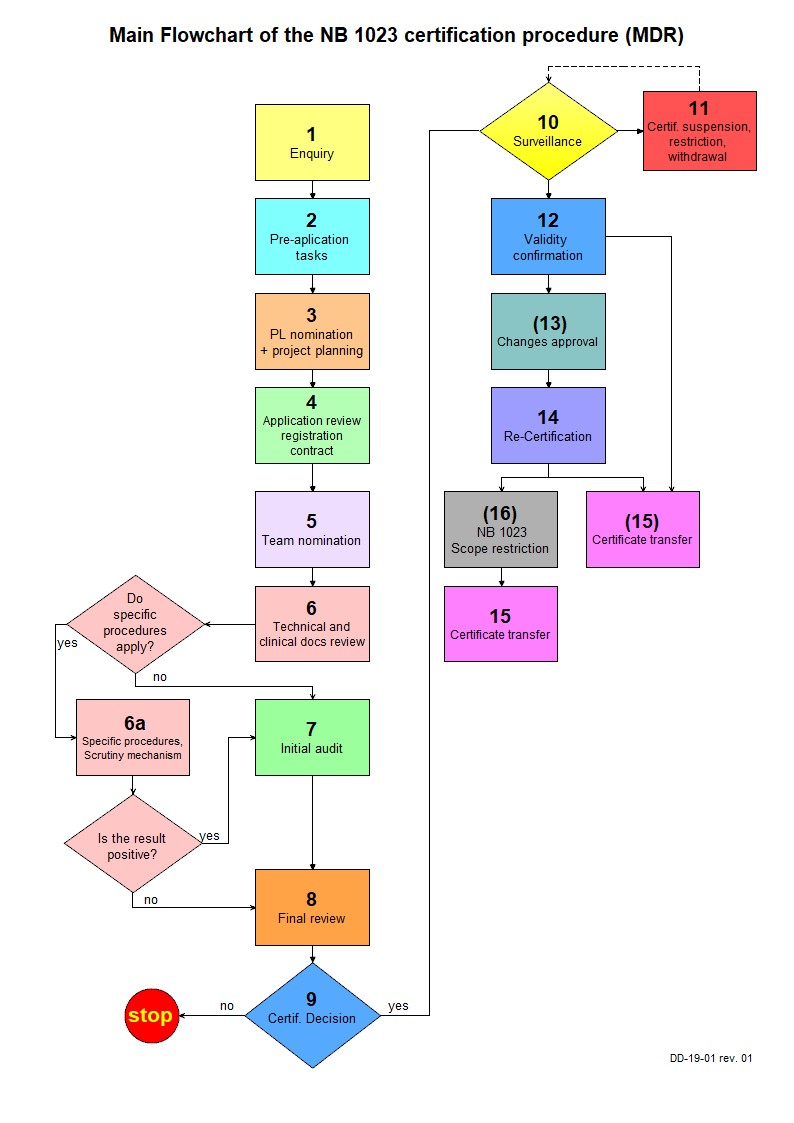
The individual steps of the application and review process by NB 1023 are illustrated in the attached process flowchart.
The languages of communication with NB 1023 staff are Czech, Slovak and English. All documentation accompanying the application must be prepared in these languages.
SCHEME OF CONFORMITY ASSESSMENT PROCEDURE (MDR)
DOCUMENTS
Following documents are requested within the initial phase of conformity assessment process.
WIDAR
The WIDAR is offline application used by NB 1023 to demonstration that requirements of MDR 2017/745 have been fulfilled. After approval of price offer and submission of an application for conformity assessment, the manufacturer receives instructions how to use WIDAR application.
FEES FOR CONFORMITY ASSESSMENT
The fees for particular conformity assessment activities are presented in following document:
CONTACT
Markéta Klinkovská
administrator
Mgr. Petr Vobejda
Head of medical device certification department
POST ADDRESS
Institut pro testování a certifikaci, a. s.
section of medical devices certification
workplace Zlín, area SVIT, building 113
trida Tomase Bati 5264
760 01 Zlin, Czech Republic


